Smart Materials for Smart Users
As polymers and polymeric materials are “the” smart invention and technological driving force of the 20th century, the quest for materials with self-regenerating properties is increasing. When polymers are degraded by thermal, mechanical and photochemical impact, self-healing serves to enhance their lifetime and thus achieves regeneration of the materials similar to living matter. The vast technological importance of these materials has had significant impact on polymer science. Prolonged lifetimes, reduced waste together with an improved consumer acceptance are expected, when self-healing properties are introduced into coatings, thermoplastic or elastomeric materials.
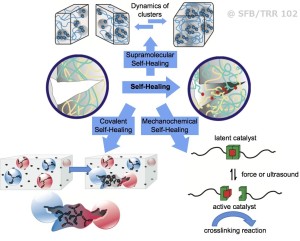
We have developed self-healing polymers based on either “click-based” chemistry, or via purely supramolecular interactions (such as hydrogen bonds or ionic interactions, see Figure 1), where cluster and network formation is among the principles. Basically three different principles are used to cause healing: The first system is based on embedded capsules, enabling to mix two reactive components after capsule rupture has occurred via mechanical force. The second concept achieves multiple healing via supramolecular polymers, able to decluster reversibly under formation of a transient network. A third concept is enabled by a direct transformation of mechanical energy into chemical energy, using so-called mechanophores. In this method, a catalyst is mechanically activated by e.g. shear-force, in turn generating an active catalyst able to perform a catalytic reaction.
Encapsulation for self-healing
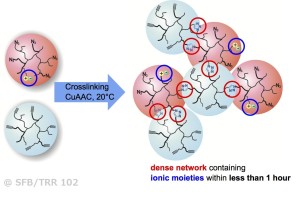
“Click”-chemistry can be used in combination with encapsulation strategies, embedded into a thermoplastic or elastomeric polymer matrix. Selection of the used chemical reactions usually takes into account the efficiency (“free energy”) of the reactants together with the stability and selective incorporation of the respective functional groups into the final material. Especially the copper-catalyzed azide/ alkyne-“click”-reaction (CuAAC) is a potentially useful chemical crosslinking reaction. The high thermodynamic gain tends to drive the reaction to completion, thus achieving a complete and dense network formation. After capsule rupture, the contents of the capsules mix, subsequently generating a network able to heal the crack. “Click”-reactions are (in contrast to many other chemical reactions) substrate independent and very efficient and enable the development of truly autonomous healing at room temperature or below. Multivalent polymers (liquid, low-Tg polymers based on polyolefins or polyacrylates) are used as highly mobile components in “click-based” self-healing materials, focusing on catalytic conditions at room temperature. Thereby we specifically address and optimize copper-catalyzed “click”-reactions by variation of the functional group density. We have discovered that especially autocatalysis arising from the newly formed triazole-moieties enables to achieve healing at room temperature within less than one hour.
Supramolecular self-healing: the dynamic strength of hydrogen bonds and ionic clusters
In contrast, purely supramolecular interactions (such as hydrogen bonds or ionic interactions) can reform, and regenerate a network with dynamic properties by itself, potentially leading to self-healing behavior. We have achieved the development of self-healing polymers displaying more than one healing cycle, which was made possible by the introduction of the supramolecular concept either via specific hydrogen bonds or ionic clusters, thus improving the design of self-healing polymers.
Not all supramolecular moieties can act as reversible “glue (stickers)” between the polymer chains: Only those acting reversibly to form cubic clusters at room temperature (e.g. the barbiturate-moiety as well as specific ionic clusters) proved advantageous in terms of healing efficiency over many other supramolecular bonds of even stronger aggregation-strength.
Despite their sometimes absolute weakness in solution, some supramolecular groups display the correct balance between chain reorganization and melt-flow of the material, thus generating a rubbery, self-healing polymer displaying multiple healing cycles. Mechanical tests of polymers with different molecular weights revealed the independency of the healing, thus proving the sole effect of the supramolecular clustering. Extension by use of ionic moieties (“ionic-liquid-polymers”) created polymers with strongly tunable flow-properties, which display enhanced diffusion and thus tunable melt flow, similar to hydrogen bonded polymers. Therefore the combination between hyperbranched polymers containing ionic liquid moieties and the “click”-based concept displays both healing concepts in the same polymer.
Future applications being tested and started in contact with industry, where the materials are implemented into elastomeric and thermoplastic coatings, but also by implantation of self-healing nanocomposites. Further work is currently dedicated to introduce mechanochemical concepts in combination with supramolecular and covalent healing concepts.